Fifteen years ago, scientists discovered a new species of deadly, drug-resistant fungus: Candida auris. It is now considered one of the most dangerous fungal pathogens on Earth. In this excerpt from “What if Fungi Win?” (Johns Hopkins University Press, 2024), author Arturo Casadevall looks at the rise of this deadly fungus, which could be the first to have emerged as a result of climate change.
In 2009, doctors at a hospital in Japan published a paper identifying a new species of fungus they’d found in the external ear canal of a 70-year-old patient. They called it Candida auris, with auris being the Latin word for ear.
At first, it wasn’t clear how much anyone should worry about this new discovery, if at all. Many new fungal species are reported in patients each year, but the overwhelming majority of these turn out to be isolated case reports, nothing worth panicking about.
And C. auris laid low for a while, remaining an obscure unknown yeast in most parts of the planet — until it was found in hospitalized patients in all corners of the world, at roughly the same time. Strikingly, between 2012 and 2015, doctors in South Africa, Venezuela and on the Indian subcontinent simultaneously reported treating patients severely ill with what turned out to be C. auris infections (remember, no one had heard of this fungal species a few years earlier).
With no contact or commonalities between them, these C. auris cases had appeared independently on three different continents, with each fungus genetically distinct from the others.
This meant that the usual suspect for fungal spread — our globalized world — wasn’t at play here. Something new was afoot. It quickly became clear that this fungus was remarkably resistant to treatment. More than one in three patients with invasive C. auris infections in their blood were dying. In hospitals where this invasive fungal disease had been nonexistent, it was now a significant cause of death.
To this day, C. auris remains mostly resistant to the antifungal treatments we have at our disposal, so once patients (and hospitals) become infected, it’s nearly impossible to get rid of. Usually, doctors diagnose fungal infections after ruling out other sources, say, when a hospitalized patient has a fever that doesn’t go away after treatment with antibiotics. Blood tests will likely indicate high white blood cell counts, another sign of an infection, but doctors often can’t tell what type of microbe is doing the damage — or necessarily know how to treat it.
Related: Potentially deadly ‘superbug’ fungus is spreading faster in the US
Symptoms will vary depending on the fungal species. Cryptococcus neoformans usually involves the brain and causes terrible headaches. Aspergillus tend to attack the lungs, meaning the patients will be coughing, short of breath and reveal something pneumonia-like on a chest X-ray.
Disease-causing fungi can be breathed in or enter the bloodstream through cuts and wounds; infections can spread in the hospital through poorly maintained IV lines and catheters. Most often, the common symptoms are fever, malaise or just not feeling well. In the hospitals where C. auris was being identified, it was mainly spreading among immunocompromised patients — despite person-to-person transmissibility being a rare quality for an invasive fungus.
It could do this because, as doctors would learn, it can colonize human skin, last for weeks on surfaces and tolerate heavy-duty hospital disinfectants. Some hospitals have reported finding C. auris spores lingering in hospital rooms long after a patient was discharged or died, even after other fungal species have been eliminated by cleaning agents. C. auris didn’t just stay overseas.
By 2016, the U.S. Centers for Disease Control and Prevention (CDC) issued an alert to healthcare professionals and labs to be on the lookout for the new pathogen. By then, a total of seven cases of drug-resistant C. auris had already been reported in hospitalized patients in four states: New York, New Jersey, Maryland and Illinois. C. auris was hard to identify by traditional lab tests, so doctors were urged to report any suspicious microbes to the CDC.
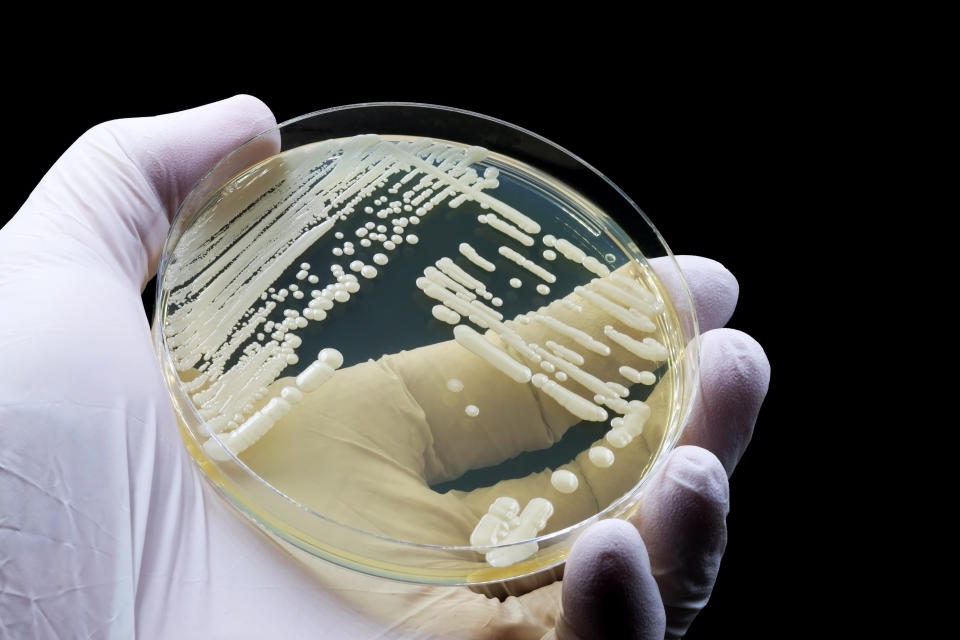
Within a decade of it being identified, C. auris was killing people in 40 countries on six continents. We don’t have exact numbers, as many cases go unreported or unrecognized, but the figure is certainly in the thousands. In the US alone, there were more than 2,000 infections in 27 states and the District of Columbia confirmed in 2022, and Mississippi reported its first cases (and deaths) of C. auris in early 2023.
Fifteen years after the fungus was discovered, the World Health Organization now considers C. auris one of the most critically harmful fungi to humans. The percentage increase in clinical cases of C. auris in the US has grown every year, from a 44% increase in 2019 to a “dramatic” 95% increase in 2021, according to a 2023 CDC study published in the Annals of Internal Medicine.
Another disturbing trend: The number of C. auris infections that were determined to be resistant to antifungals in 2021 was about three times more than in each of the previous two years. As the authors note, “screening is not conducted uniformly across the United States, so the true burden of C. auris cases may be underestimated.”
The march of drug-resistant C. auris clearly isn’t slowing. If anything, it’s rapidly speeding up. How did a fungus, so deadly to immunocompromised humans, just suddenly appear? And why was it killing people when it had never even made people sick before? Where did it come from? It is both mysterious and alarming.
After the discovery of C. auris, teams of researchers went back and explored old fungal samples. They determined that the fungus had likely been around since the late 1990s in some form, but they found no sign of C. auris prior to that and no evidence that it had ever killed anyone before.
C. auris, of course, didn’t just show up on the Earth in the last 30 years out of nowhere. This ancient life form was certainly living in the environment long before that, shown by the fact that it had evolved into several different strains by the time it was identified. Yet it had been invisible to humans. Since C. auris hadn’t been considered medically relevant until the 21st century, no one was looking for it.

There was no need. Until there was. “We know that new species don’t just appear,” Shawn Lockhart, a CDC researcher, told JAMA in 2022. “We just have not figured out where it was hiding before it started to appear in hospitals worldwide. There’s a lot of speculation.” Lockhart’s best guess is that C. auris quietly coexisted with humans in the ear canal — until it turned up the volume. Body temperature may have long protected us from fungus, but we would quickly learn that C. auris was different. Unlike most of its fungal cousins, it can grow at temperatures as high as 42 degrees Celsius (107.6 degrees Fahrenheit).
This is what makes it so deadly to some humans with weakened immune systems. On some level, we haven’t appreciated the protective role of temperature in fungal infections because most of the viral and bacterial diseases that currently plague animals and humans are typically acquired from other warm hosts — meaning they’re already adapted to mammalian temperatures and can be transmitted with relative ease. For example, we catch the flu, COVID-19, tuberculosis and more from other humans (and perhaps other mammals), and those microbes are accustomed to replicating at our higher body temperatures.
RELATED STORIES
—Plastic-eating fungi could help take a bite out of Earth’s rampant pollution crisis, study suggests
—Cause of mysterious brain-invading-fungus outbreak finally discovered
—‘Few insect orders have been spared’: Why death by parasite keeps life in the forest thriving
But things change when you focus on environmentally-acquired microbes, particularly fungi. While many fungi still can’t survive at normal human temperatures, some can and do. C. auris is Exhibit A. In a 2010 paper, published in the journal mBio, microbiologist Mónica García-Solache and I had predicted that a fungal threat like C. auris would be coming, though we didn’t yet know its name.
“Global warming could have a significant effect on fungal populations,” we wrote. “First, a warmer climate could change the distribution of heat-tolerant and susceptible species by favoring those that are more thermotolerant, and by creating conditions for more environmental fungi to spread and enter into closer contact with human populations. Second, under strong selective pressure, the prevalence of species adapted to heat tolerance may increase.”
It wasn’t a matter of if fungi would adapt to temperatures high enough that they could threaten humans, we warned, but when.
Excerpted from What If Fungi Win? by Arturo Casadevall, MD, PhD, with Stephanie Desmon, MA. Copyright 2024. Published with permission of Johns Hopkins University Press.


What if Fungi Win? by Arturo Casadevall — $16.95 on Amazon
Humans and fungi share nearly 50 percent of the same DNA. Because we’re related, designing drugs to combat the varieties that attack us is a challenge. Meanwhile, in an ever hotter, wetter world, fungi may be finding new ways to thrive, queueing up global outbreak potentials for which no vaccine and woefully few medications exist; some fungi are already beginning to resist treatment. Among other lifeforms, bats, amphibians, and essential crops are also increasingly threatened by these pathogens.
Enter fungal kingdom frontiersman Dr. Arturo Casadevall, an epidemiologist, professor, and inventor. Casadevall shares how the 1990s AIDS epidemic’s fungal complications drove his medical mycology work, how COVID-19’s fungal incidences underscore the continuing threat to the immunocompromised, and how he and his Johns Hopkins University laboratory team are discovering ways to counter the threats posed by these cunning, hungry combatants.View Deal
Source Agencies


